The client approached us with the ask to empower compliance with healthcare regulators and ensure that use of product documentation becomes auditablesacross all clinical studies. In order to understand the magnitude of the challenge, identify the right business areas to reimagine, and proof the potential value of a future solution, my team and I have taken an explorative approach. The project was planned and delivered fully remote.
Research and Safety tool for Roche
Engagement Lead
Project description & approach
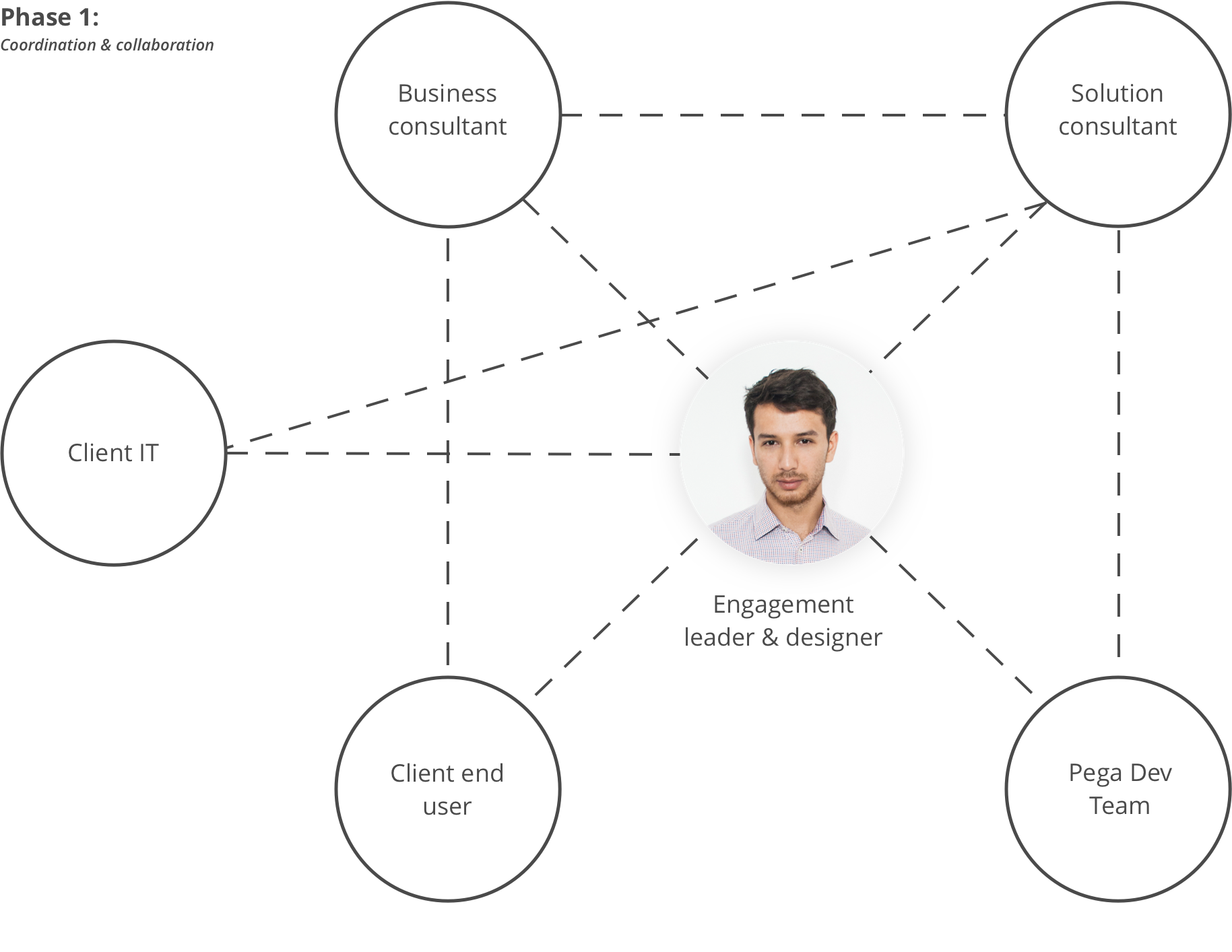
With a core team, consisting of a senior solution and a senior business consultant, I have framed the opportunity in order to create actionable work streams and orchestrate the project. At the solution creation phase, we scaled the team to deliver a proof of concept and receive approval for solution build. In the second phase of the project, I have initially led an interdisciplinary team of consultants, client stakeholders and designers until the design for the MVP was production-ready.
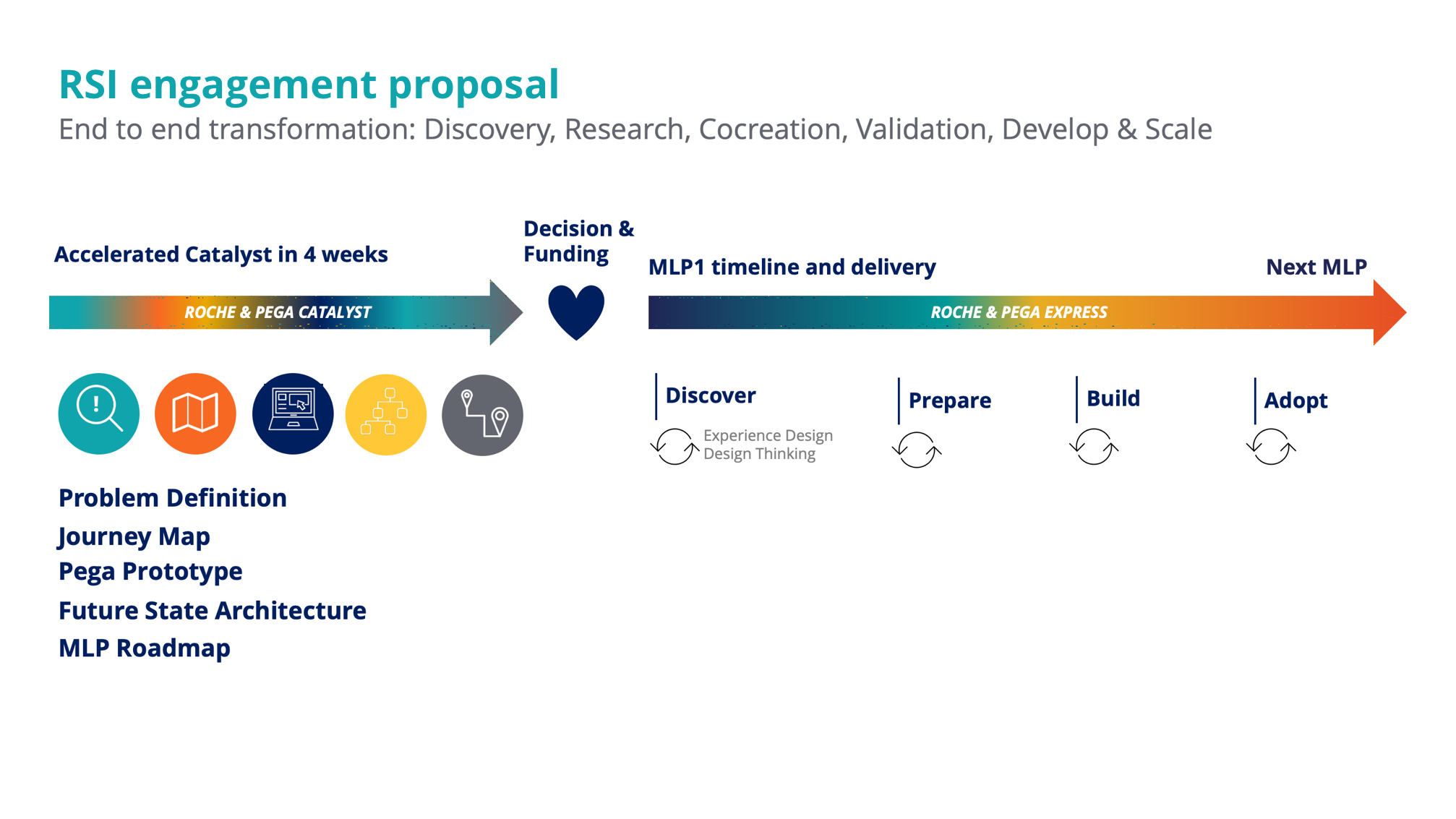
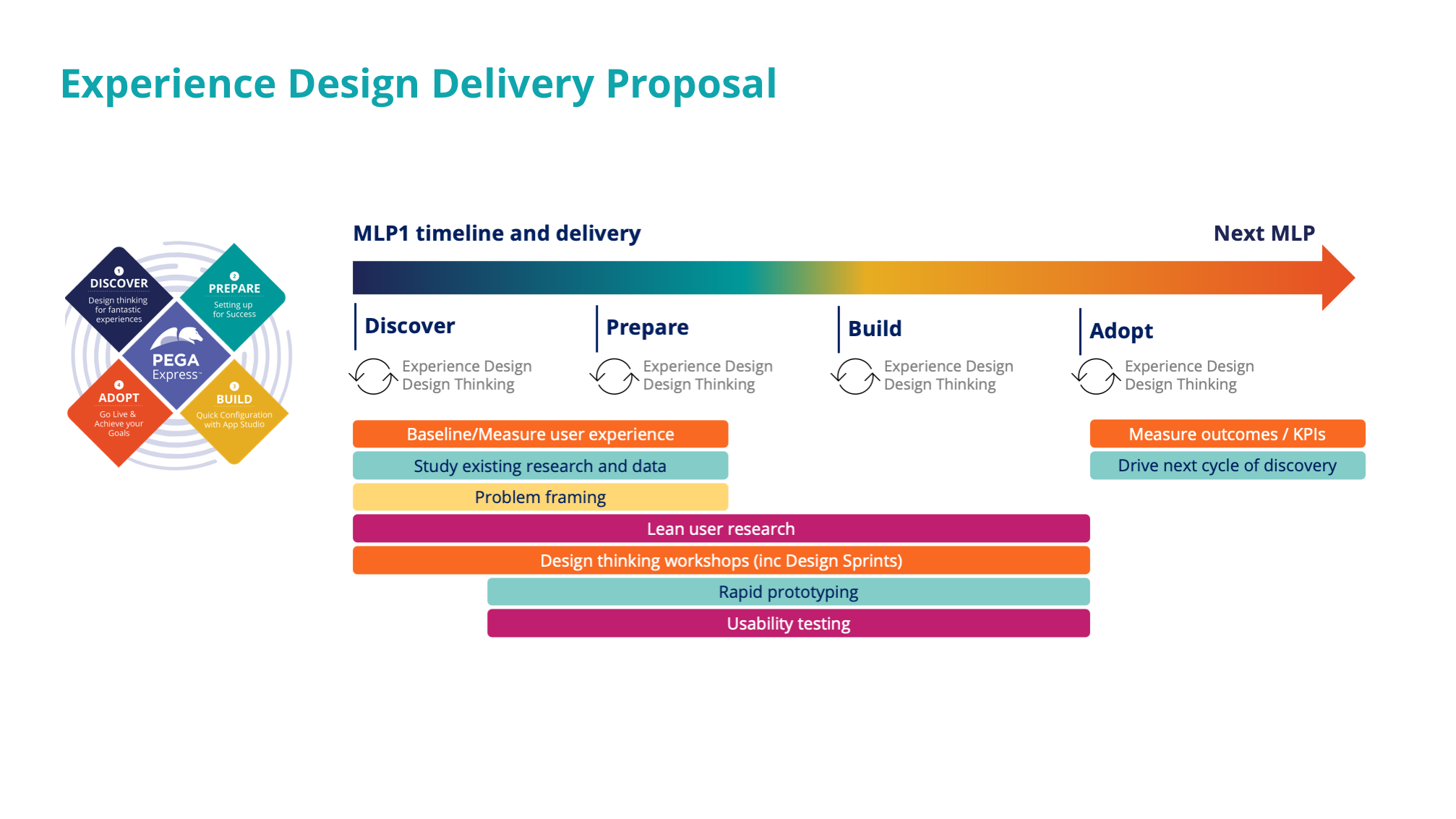
Phase 1 | Catalyst approach
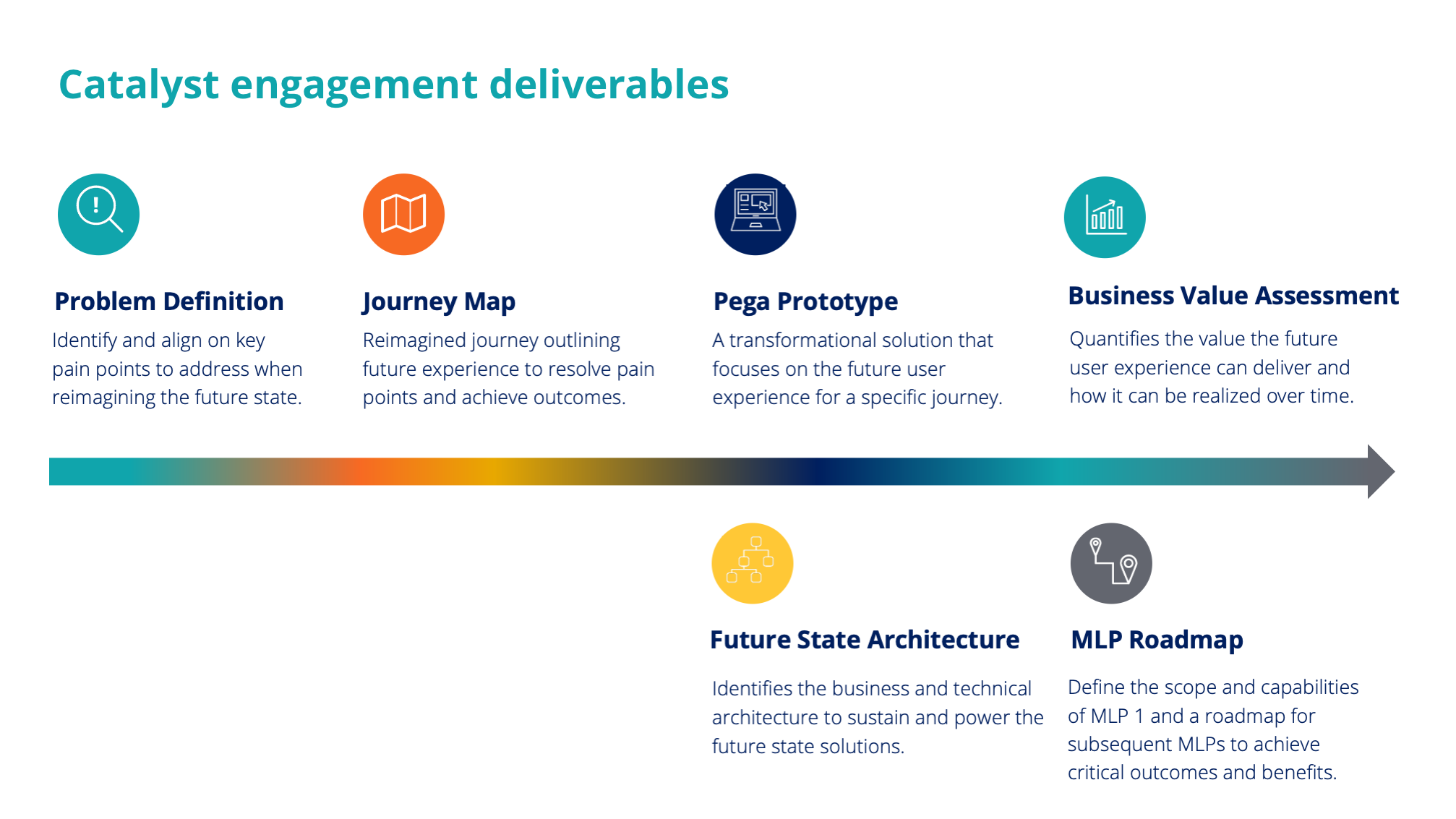
Design activities & deliverables
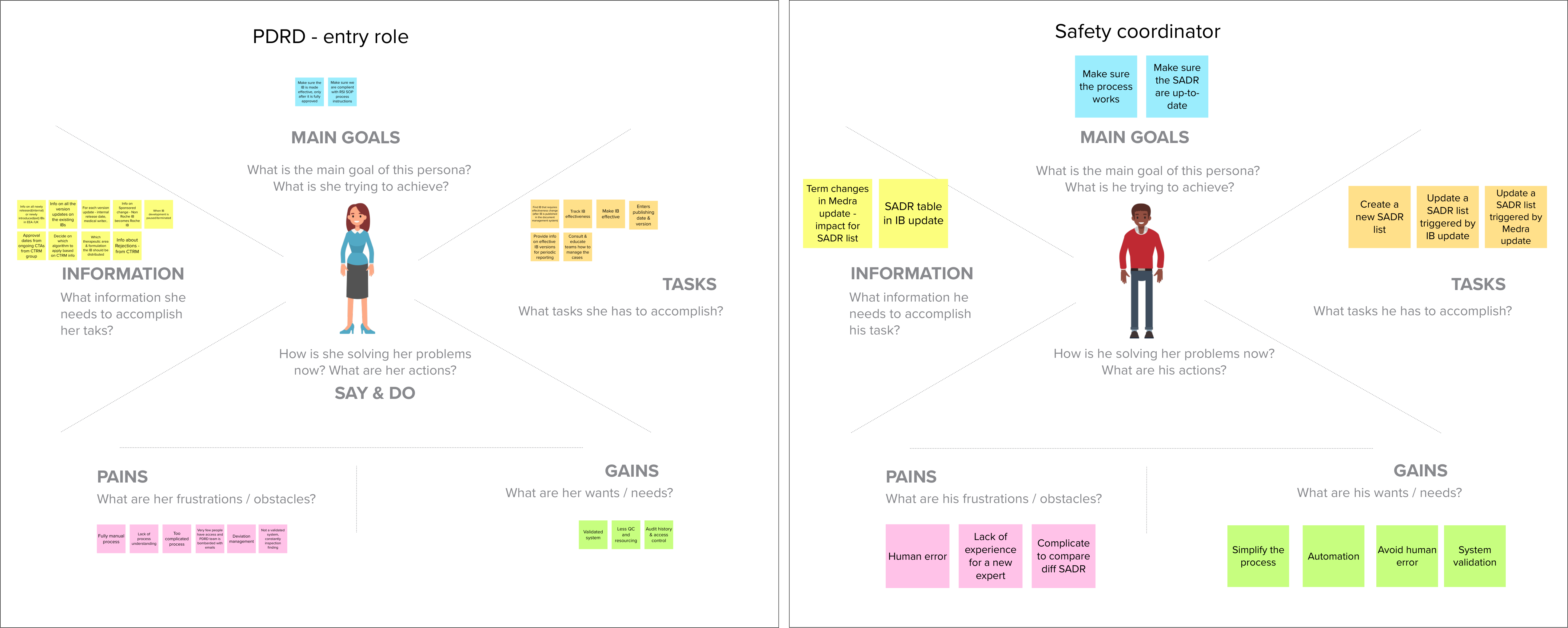
Research & problem framing
We conducted various interview, framing and walkthrough sessions to obtain a macro-view on the business area, understand the underlying clinical-trial processes, analyse stakeholders and their needs and identify personas and pain points.
The produced insight were harnessed to empower the client´s business-IT team, and instigate decision-making to transform this area of business.
Focusing on innovation areas
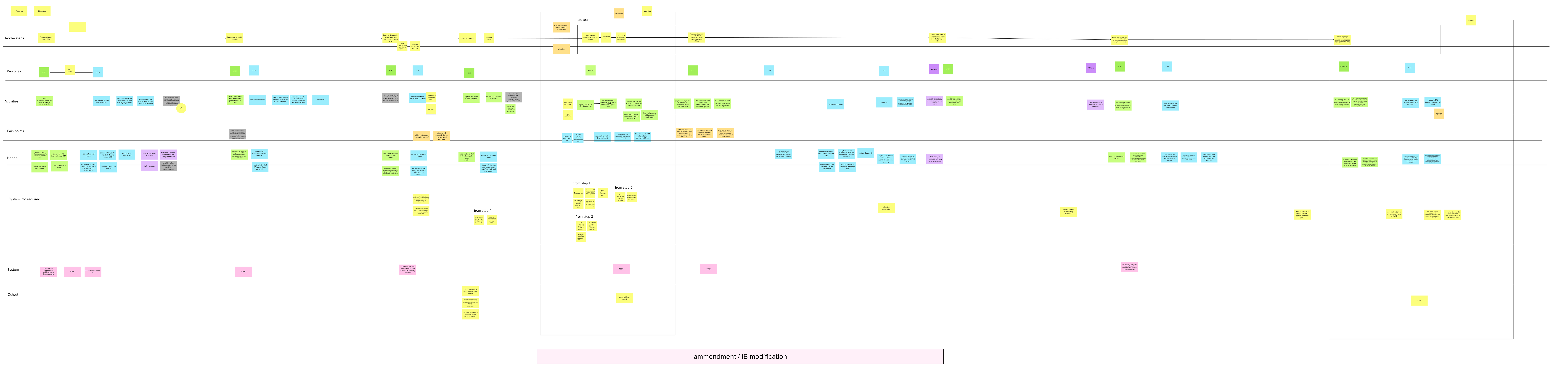
Once we understood the end-to-end process, we identified and zoomed-in on the critical areas for compliance and audit. This allowed us and the client team, to determine the initial scope for the future solution and served as starting point for prototype design and development.
Prototype development
For the selected focus areas we redesigned the process to become more efficient by streamlining and automating process steps. Further, we incorporated capabilities to become fully auditable. Based on a story board, a team of 4 developers, delivered a functional proof of concept with real customer data, within 2 weeks time.
Phase 2 | Delivery
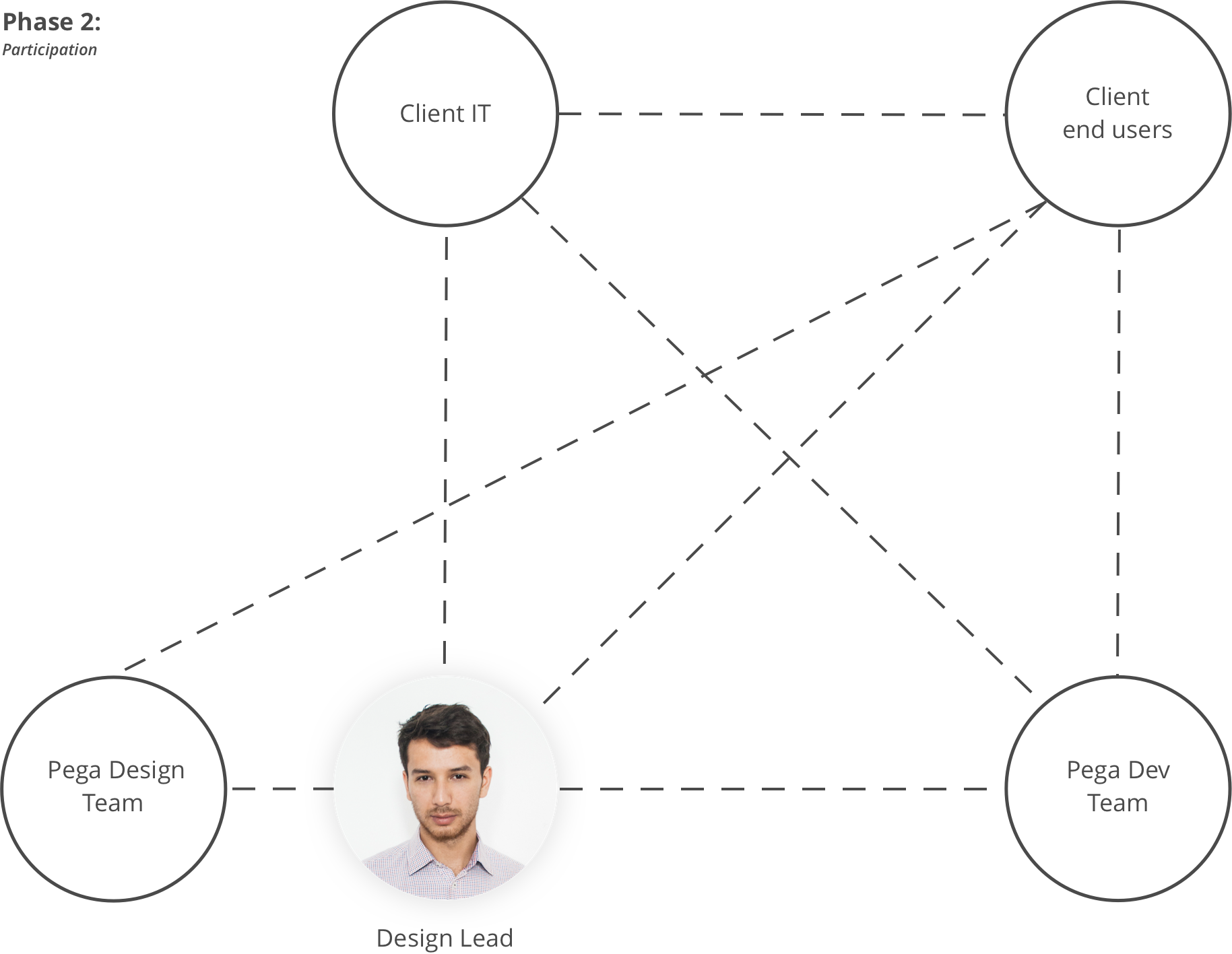
Lead & Designer
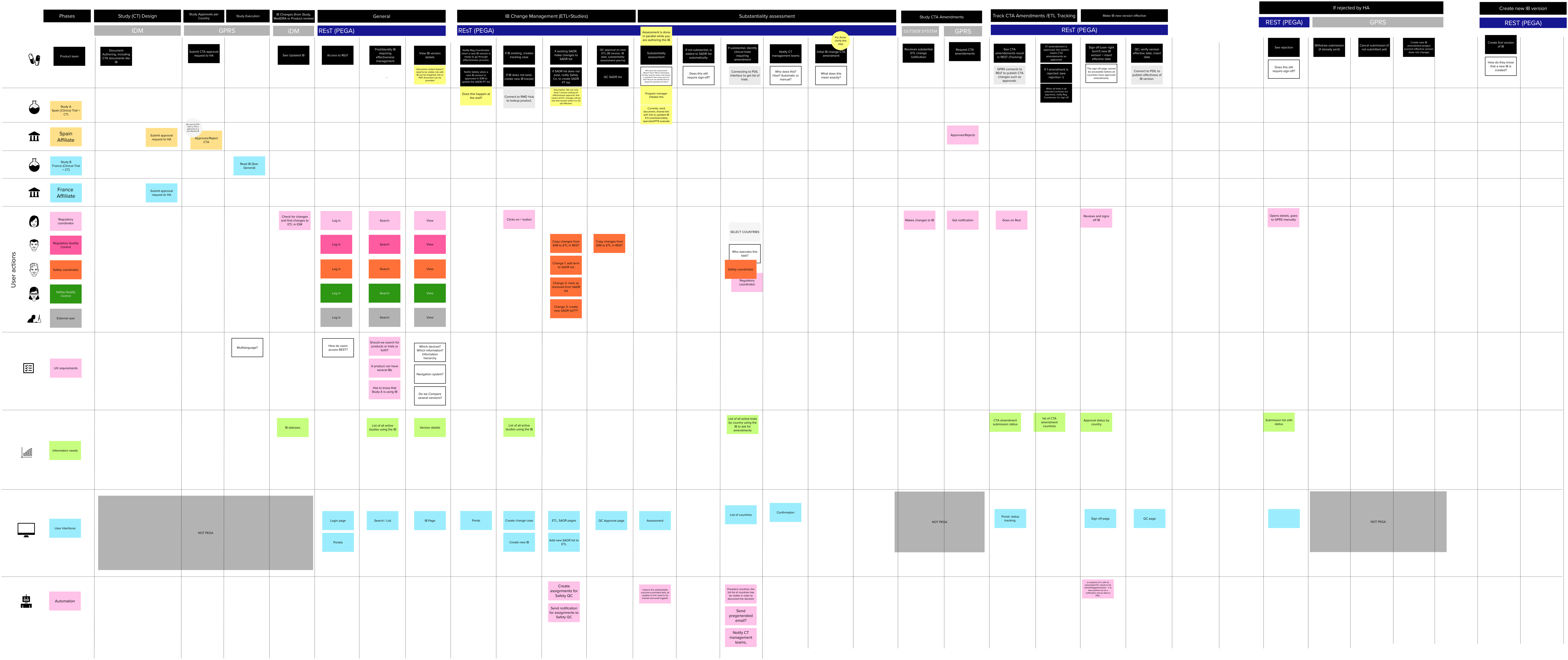
System Journey
After the PoC, the scope of the solution was extended to 4 user roles and 3 functional areas. To design all user-interactions for such a complex system, we took a system-focused approach to satisfy all stakeholder needs.
Through multiple co-creation sessions with end-users and IT stakeholders, we designed the future end-to-end business process, determined system artifacts and defined the integration interfaces with other systems.
Team set-ups
Phase 1 & Phase 2
Product Design
Design Lead
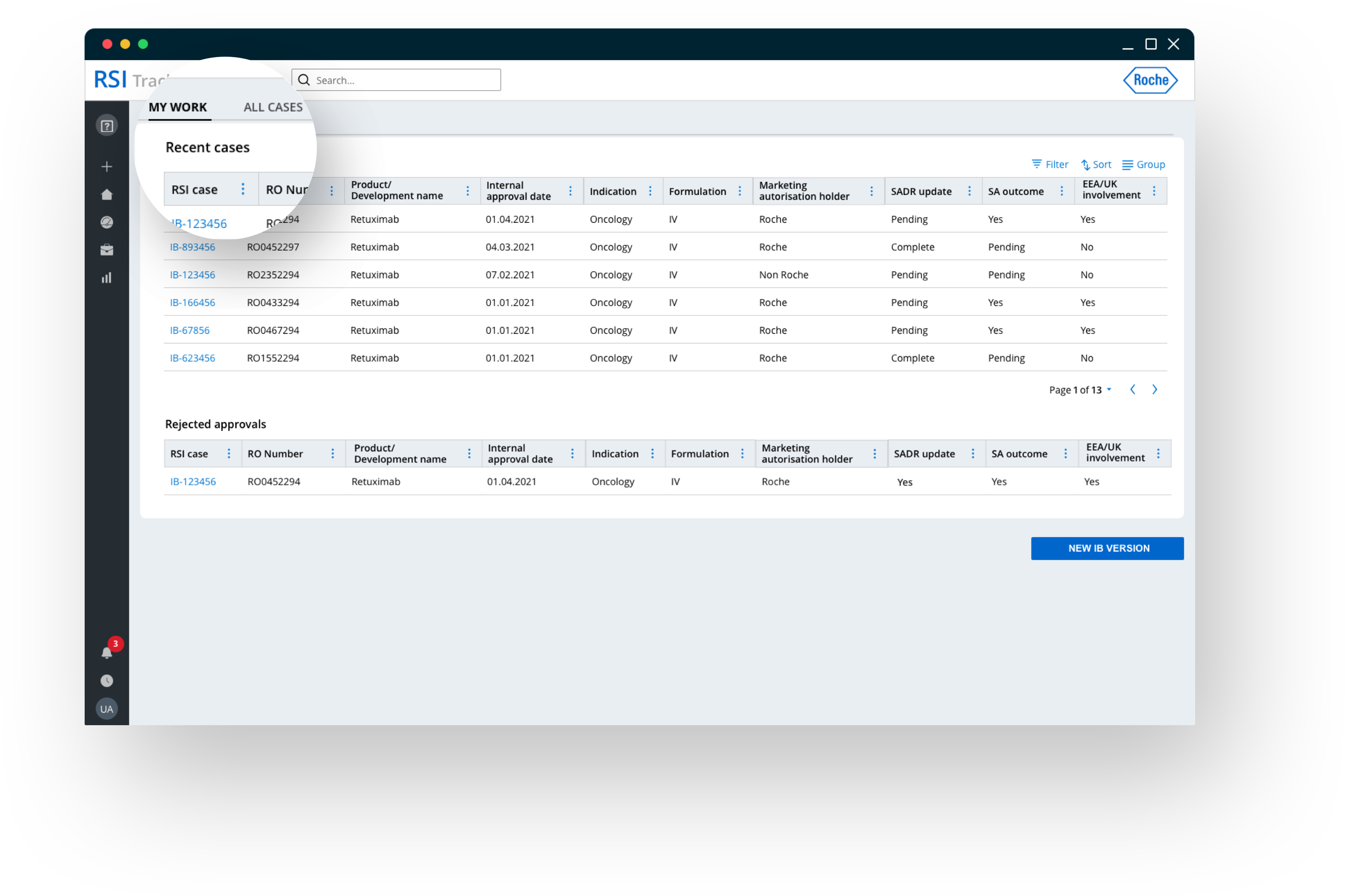
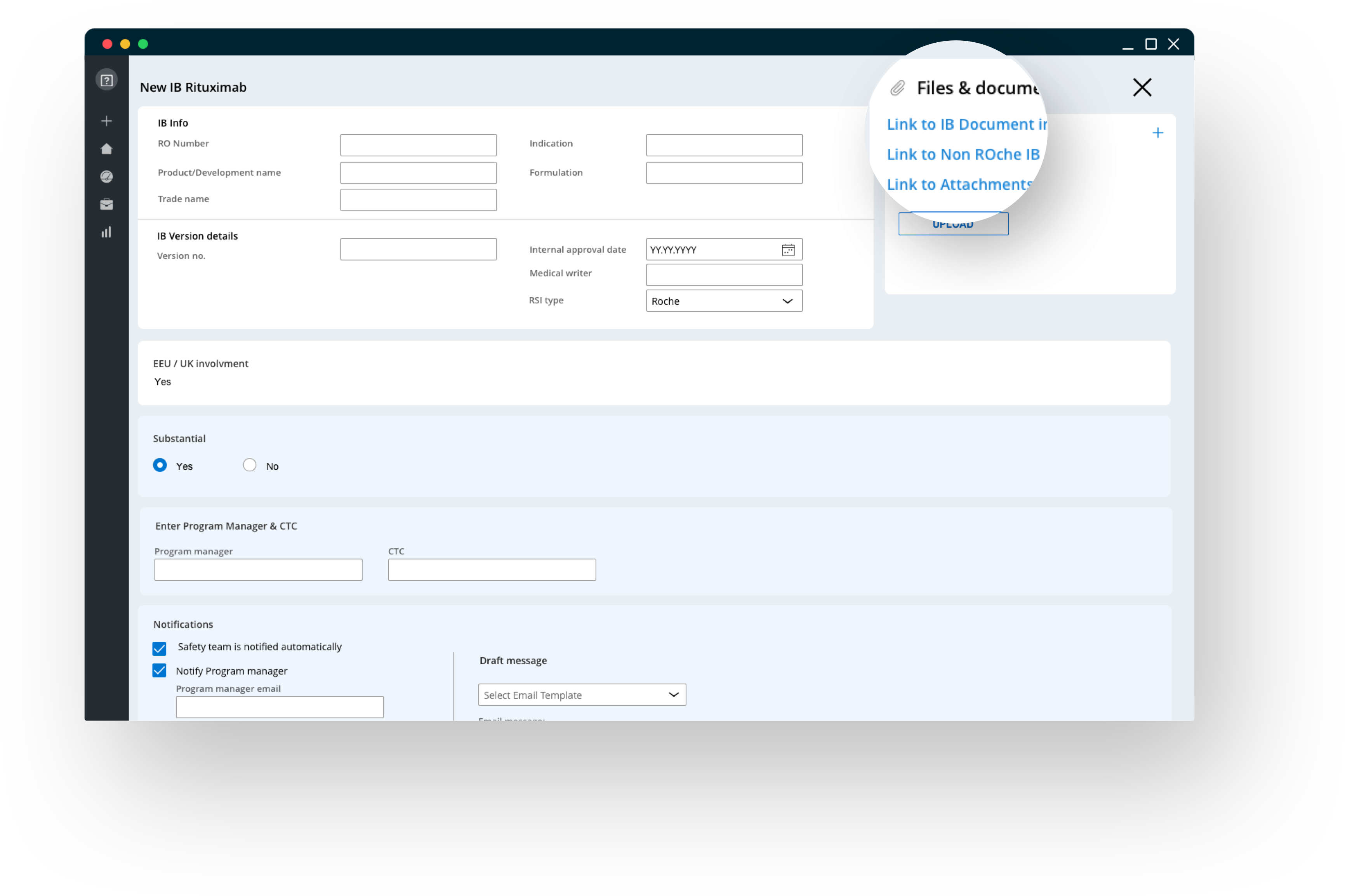
Full business object management with real-time collaboration
Workflow management to create, edit, version, submit and track IBs across all running clinical studies, provides end-users with a 360° understanding on current IB status. The solution supports collaboration along the whole business process, ensures end-to-end auditability and allows to easily adjust the global workflow, when necessary.
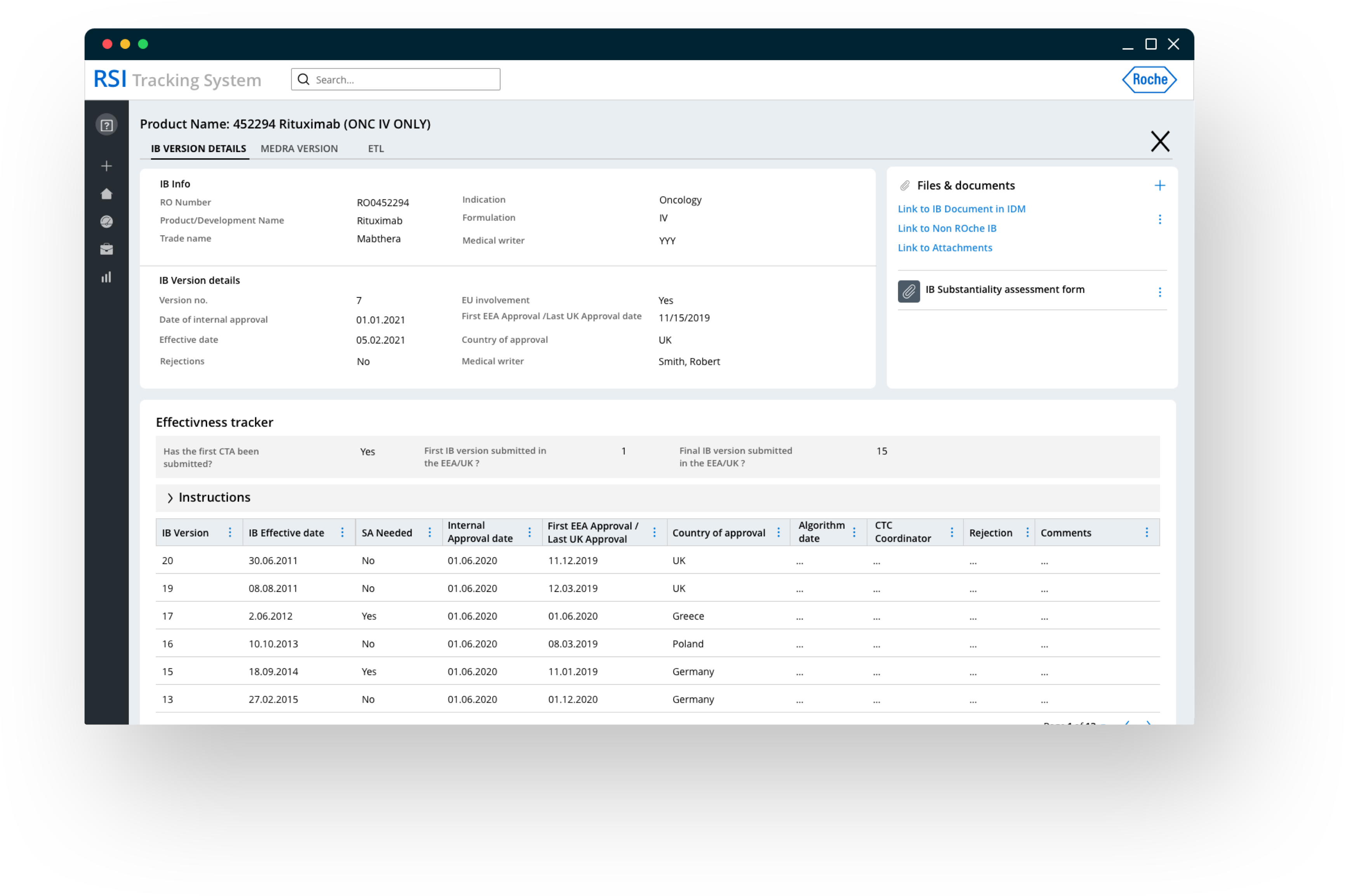
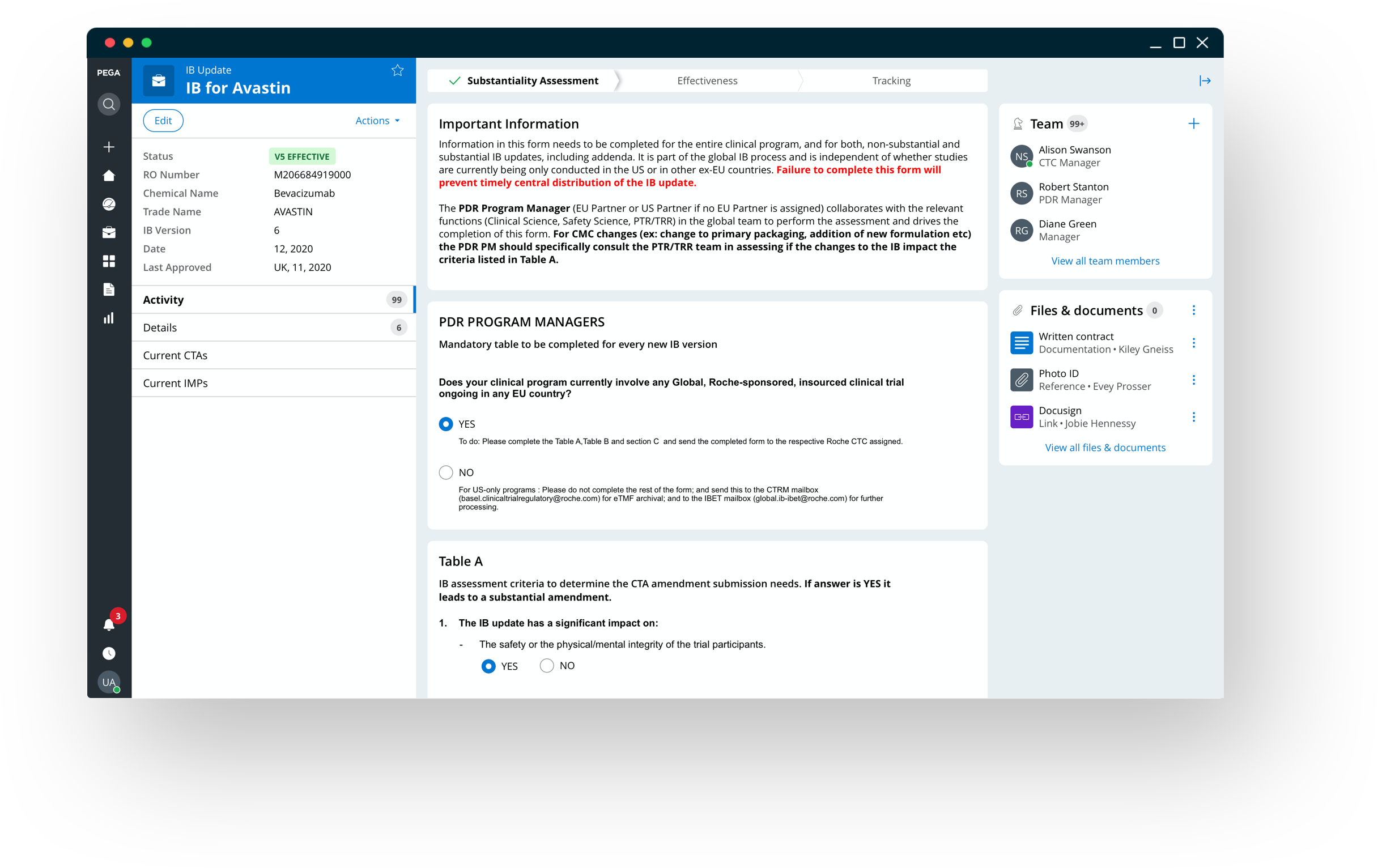
Managing object effectiveness & ensuring business continuity through a single source of truth
Integrating with the existing trial management system, empowers the organization manage the whole IB lifecycle from development to application until termination.